
Start a New Era in Clinical Practice with tVNS®
A drug-free and non-surgical solution that improves your quality of life:
tVNS® is a scientifically proven treatment that supports body balance by non-invasively stimulating the vagus nerve through the ear.
This innovative system, which applies precise stimulation to the cymba concha region, offers personalized therapy with wireless parameter settings.
Approved as a Class IIa medical device under the European Union Medical Devices Directive (EU-MDR), tVNS® meets the highest standards for safety and effectiveness.
tVNS® R
Transcutaneous Vagus Nerve Stimulation Device
Full Wireless Parameter Programmability
Patient Application for Data Collection
Support for Clinical Research
Triggered tVNS®
Legacy and Hook Electrode
Sham Stimulation
Leading Healthcare Organizations Worldwide Trust tVNS® Devices

































Why Are We Pioneers in This Field with tVNS®?
We Offer Reliable and Scientific Solutions with Over 20 Years of Experience
For over twenty years, we have been pioneering the field with our innovative work in non-invasive vagus nerve stimulation.
Our devices were developed in Germany using advanced technology, and their effectiveness has been validated through clinical studies. Approved as a Class IIa medical device under EU-MDR, tVNS® stands out in the clinical field not only for its effectiveness but also for its reliability and low side effect profile. In today's world, where scientifically unverified practices are becoming increasingly common, tVNS® offers an evidence-based and approved treatment method.
Since we first introduced the Cerbomed Nemos® device, we have maintained our market leadership in vagus nerve technologies. tVNS® systems, preferred both clinically and in research, distinguish themselves with their safety, precision, and user-friendliness.
Prioritizing patient comfort and long-term benefits, our devices are manufactured to the highest quality standards at our production center in Germany. Because we believe in the safe start of the healing process.
What is tVNS®?
Non-Invasive Vagus Nerve Stimulation in the Treatment of Neurological Diseases
In cases where pharmacological or invasive treatment approaches are inadequate or cause undesirable side effects, tVNS® offers an effective and comfortable treatment option for patients.
The vagus nerve, which plays a central role in our body's nervous system, is critical for maintaining internal balance (homeostasis). tVNS® (Transcutaneous Vagus Nerve Stimulation) harnesses the therapeutic potential of this nerve by targeting its auricular branch, which is accessible in the ear.
This innovative method modulates vagus nerve activity with low-intensity electrical stimulation and aims to promote balance among body systems. This reduces the symptoms of neurological disorders associated with sympathovagal imbalance. Furthermore, this effect is achieved without the need for surgery or the side effects of medications.
Approved as a Class IIa medical device under the European Medical Device Regulation (EU-MDR), tVNS® sets a new standard by combining efficacy, safety and ease of use in the treatment of neurological diseases.

tVNS® is indicated for the treatment of the following clinical conditions:
✓Anxiety
✓Atrial Fibrillation
✓Autism
✓Cognitive Disorders
✓Depression
✓Epilepsy
✓Crohn's Disease
✓Inflammatory Bowel Disease
✓Migraine
✓Parkinson
✓Prader-Willi Syndrome
✓Sleep Disorders
✓Systemic Sclerosis
✓Tinnitus
Why Choose tVNS® Device?
tVNS®: The Only Non-Invasive Vagus Nerve Stimulation Device with EU-MDR Approval
tVNS® is the only non-invasive vagus nerve stimulation device approved as a Class IIa medical device under the European Medical Device Regulation (EU-MDR).
This innovative, clinically validated, non-surgical method offers a safer and more comfortable alternative to invasive procedures.
Using patented electrode technology, it provides maximum effectiveness by stimulating the concha cymba, targeting the auricular branch of the vagus nerve (100% innervation). Unlike other devices that only provide partial innervation, tVNS® is a unique solution that maximizes effectiveness in this regard.
EU-MDR Approved Medical Device
tVNS® is the only non-invasive vagus nerve stimulation (VNS) device approved under the European Medical Device Regulation (EU-MDR).
Our device has a unique stimulation technology that targets the cymba concha region, providing 100% innervation of the auricular branch of the vagus nerve.
Unlike other devices on the market, they provide partial innervation by stimulating different anatomical areas and are not manufactured to the same quality and safety standards as tVNS®.
Non-Surgical Clinical Approach
tVNS® provides the clinical effectiveness of vagus nerve stimulation without the risks and operational difficulties associated with surgical interventions.
In this way, patients can benefit from significant therapeutic benefits in a non-invasive way and under their own application control.
Minimal Side Effects
The process of moving away from pharmacological treatments often goes hand in hand with the search for fewer side effects.
tVNS®, a drug-free method, offers a safer treatment alternative by reducing the risk of long-term adverse effects.
German Engineering and Production Quality
Our commitment to excellence ensures that every tVNS® device meets the highest standards for quality and performance.
Our devices are developed with durability and innovative design in mind, reflecting Germany's long-established engineering and manufacturing precision.
With tVNS®, you invest in a technology focused on clinical success, trusted by healthcare professionals and researchers worldwide.
Mobile App-Supported Therapy Experience
Tracking and understanding your treatment progress is critical to clinical success.
The tVNS® Patient mobile application developed for this purpose not only provides real-time monitoring but also enables seamless sharing of data with healthcare professionals.
Access from Anywhere
In today's world, remote access is of great importance for patient comfort.
In response to this need, tVNS® offers a combination of device delivery directly to the patient's address and distance learning support.
In this way, patients can easily begin their treatment process without the necessity of travel or logistical restrictions.
Pediatric Compatibility
tVNS® has been rigorously designed and approved for use in children.
In this way, it fills an important gap in pediatric treatment in a safe and effective manner.
Easy to Use
Functionality should not compromise on simplicity.
Standing out with its advanced technology, the tVNS® device is also as user-friendly as a smartphone and can be effortlessly integrated into your daily routine.
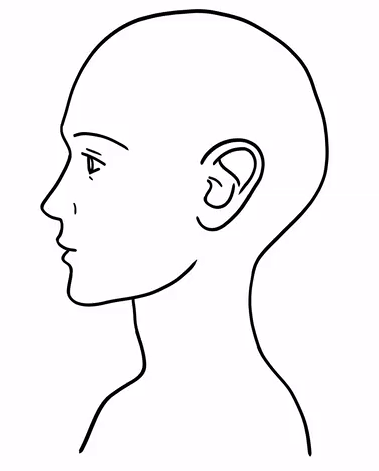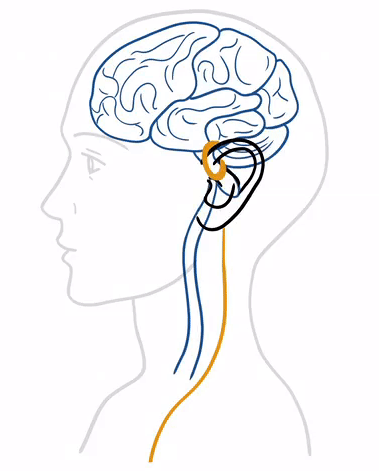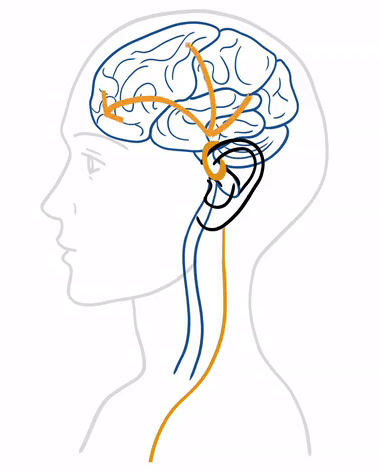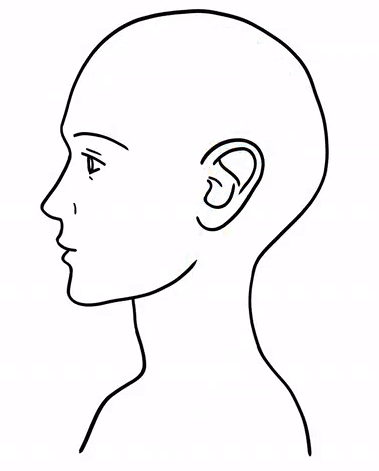

tVNS® treatment stands out for its ease of use and integration into daily life . At the heart of this method is the principle of neurostimulation, which utilizes the vagus nerve's ability to influence various bodily functions and systems.
tVNS® stimulates the auricular branch of the vagus nerve (ABVN) with gentle electrical impulses delivered through the skin. These impulses generate therapeutic signals that are transmitted first to the brainstem and then to higher centers, aiming to alleviate disorders within the nervous system.
The device is designed with a completely patient-centered approach: easy to use, portable, and discreet. This allows you to integrate treatment sessions into your schedule without disrupting your daily routine. Whether you're at home, at work, or traveling, tVNS® offers a consistent and accessible solution for managing the symptoms of neurological disorders, including reducing seizure frequency in epilepsy.
Each session with the tVNS® device is a step toward rebalancing the nervous system. This method uses the body's natural pathways to support healing and symptom relief. The device's ease of use makes starting and maintaining treatment extremely convenient. This empowers users to take control of their health through a non-invasive and effective method.
Mechanism of Action of tVNS® Therapy
tVNS® is designed to be portable and easy for patients to use at any time of the day and in any environment they choose.
tVNS® Clinical Research and Patient Tracking Application
Two Apps Integrated with Your tVNS® R Device: tVNS® Research and tVNS® Patient Your tVNS® R device provides access to two applications developed for research and patient monitoring: tVNS® Research and tVNS® Patient.
The tVNS® Research app allows all technical parameters to be easily reprogrammed and transmitted wirelessly to the device. This gives researchers the flexibility to experiment with entirely new stimulation protocols. Technical and patient data can also be collected and securely stored through the app.
The tVNS® Patient app automatically records all stimulation data (application time, duration, parameters, etc.). Additionally, it offers a structured survey module where patients can provide feedback on their experience.
The tVNS® Patient app is fully compliant with the General Data Protection Regulation (GDPR). This means patient data is processed and stored in accordance with the highest confidentiality and security standards.

tVNS® Technology Supported by Clinical Research
Multi-center clinical studies and scientific research demonstrate the proven efficacy and safety of tVNS®.
Thanks to our unique stimulation approach focused on the cymba concha region, our high quality standards, and our strong clinical data base, tVNS® has become a preferred neurotherapeutic solution for researchers.
This holistic approach positions tVNS® at the forefront of innovation and patient-centered care in the field of neuromodulation.
.jpg)
Epilepsy
After 20 weeks of treatment, patients receiving active tVNS® had an average 34.2% reduction in seizure frequency.
Prader-Willi-Syndrome
After 12 months of treatment, 80% of patients treated with tVNS® had an average 50% reduction in the number of daily seizures/attacks compared to pre-treatment levels.
Depression
tVNS® administration, when used in conjunction with psychopharmacological treatment and psychotherapy, resulted in a reduction of at least 2–3 points in depression-specific HAMD scores in adult patients after a 9-week period.
Migraine
tVNS® treatment resulted in a 50% reduction in the frequency of migraine days after 12 weeks of treatment.

Enter a New Era in Patient Treatment with tVNS®
Discover how tVNS® can transform your treatment approach. Incorporate this innovative solution into your practice, offering a safe and non-invasive way to heal your patients who haven't seen satisfactory results with conventional treatment methods. Let's take a step together to pioneer an innovative and people-centered approach to healthcare. Contact our expert experts to learn more about how you can integrate tVNS® into your clinical practice and the benefits it can provide for your patients. Together, let's pave the way for a healthier future and a higher quality of life.


.png)